A vibrant area of our research concerns the mechanics of cells and of the angiogenesis, in strong cooperation with the Patient-based and preventive medicine (MPP) lab @ UNIBS. The formation of new blood vessels is a critical part of tissue and tumor growth, as well as of healing processes. Understanding the underlying mechanisms of this process and how it affects the perfused tissues is fundamental. For example,
control of angiogenesis could help bones and tissues to heal faster or more successfully. In contrast,
the inhibition of angiogenesis in some cases could stop the development of unwanted tissues, such as a tumor, or slow the healing process for better results.
Controlled angiogenesis is also important for engineering of new tissues, or possibly whole organs needed to replace damaged ones. Deformations and mechanics of cells play a fundamental role in the angiogenesis, and we have been studying the vascular endothelial growth factors relocation and localization, towards local delivery of drugs in cancer treatments.
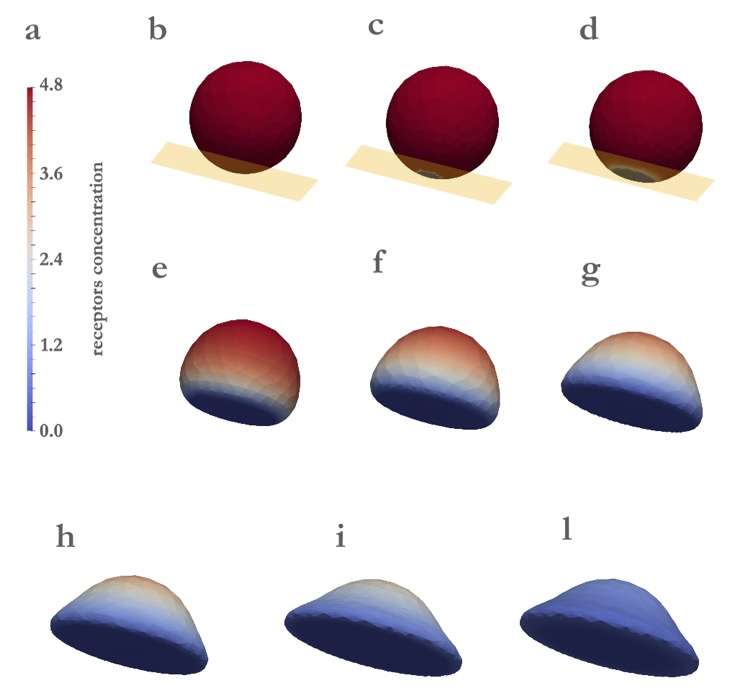
Vascular Endothelial Growth Factor Receptor-2 (VEGFR2) is a pro-angiogenic receptor, expressed on endothelial cells (ECs).
Although biochemical pathways that follow the VEGFR2 activation are well established, knowledge about the dynamics of
receptors on the plasma membrane remains limited. Ligand stimulation induces the polarization of ECs and the relocation of
VEGFR2, either in cell protrusions or in the basal aspect in cells plated on ligand-enriched extracellular matrix (ECM). We
develop a mathematical model in order to simulate the relocation of VEGFR2 on the cell membrane during the mechanical
adhesion of cells onto a ligand-enriched substrate. Co-designing the in vitro experiments with the simulations allows identifying
three phases of the receptor dynamics, which are controlled respectively by the high chemical reaction rate, by the mechanical
deformation rate, and by the diffusion of free receptors on the membrane (see video below).
The equations that govern the problem will be written in a strong dimensionless form as well as in a weak form suitable to be discretized and implemented in a finite element code.
We make extensive use of the high performance computational library
deal.ii (C++ software library supporting the creation of finite element codes), with the ultimate goal of predicting the conditions that trigger angiogenesis.
The identification of the laws that regulate receptor polarization opens new perspectives toward developing innovative anti-angiogenic strategies through the modulation of EC
activation.
In this realm, the amount of uncertainties and unmeasurable parameters is high, and it calls for tailored techniques of uncertainty quantification that are in progress.